Beware: Same Ingredients, Different FDA Indications
Wednesday, November 3, 2021
(0 Comments)
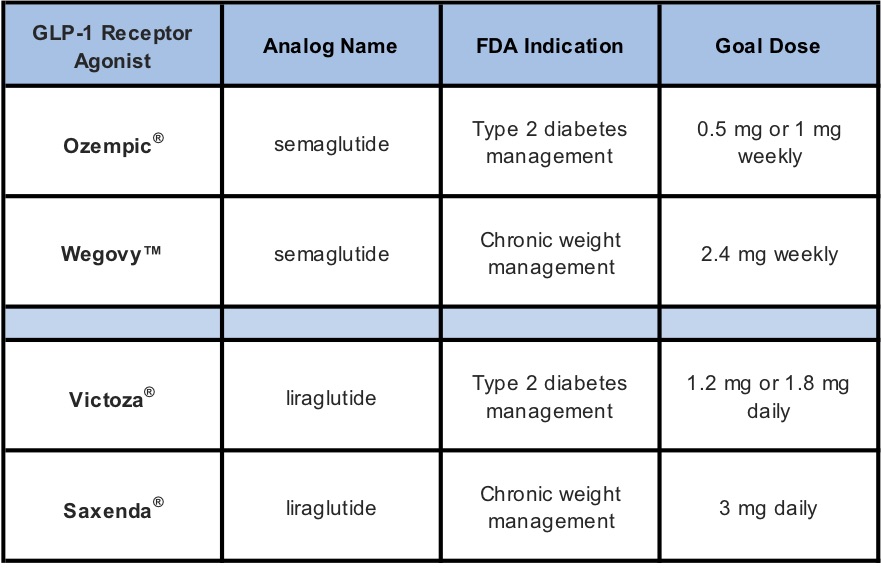
Ozempic® is an injectable diabetic medicine used to improve glycemic control in adults for type 2 diabetes management. On June 4, 2021, the FDA approved a new drug treatment for chronic weight management called Wegovy™ which has the exact same ingredient
– semaglutide.
Similarly, Victoza® is also used to improve glycemic control in patients 10 years and older for type 2 diabetes and is composed of an ingredient called liraglutide. Saxenda® also contains liraglutide but is used for chronic
weight management. Pharmacies need to be aware that there is an audit risk if the prescriber is ordering for off-label use.
PAAS Tips:
· Prescribers may try to help patients get around plan exclusions for weight loss medications by prescribing Ozempic® or Victoza® at higher doses o FDA approved medications being used off-label like this would likely not be covered under federal programs and are subject to audit recoupment o Claims paid at point-of-sale do not guarantee payment · If a prescription is written for the Analog name, pharmacies need to confirm the diagnosis to bill the correct medication · These products come in multiple strengths and package sizes which would also need to be confirmed · Medicare Part D and Medicaid may restrict coverage of medications if the prescribed use is not for a medically accepted indication · PAAS Audit Assistance members can view our May 2021 Newsline article, New Package Size Available for Ozempic® for reference on how to bill for Ozempic®.
PAAS National® is committed
to serving community pharmacies and helping keep hard-earned money where it belongs. Contact us today at (608) 873-1342 or info@paasnational.com to see why membership might be right for you.
By Trenton Thiede, PharmD, MBA, President at PAAS
National®, expert third party audit assistance and FWA/HIPAA compliance.
©2021 PAAS National® LLC All Rights Reserved
|